The fight against Kovid-19 has taken a historic leap since the first Pfizer / Bioentech vaccine was given outside a clinical trial.
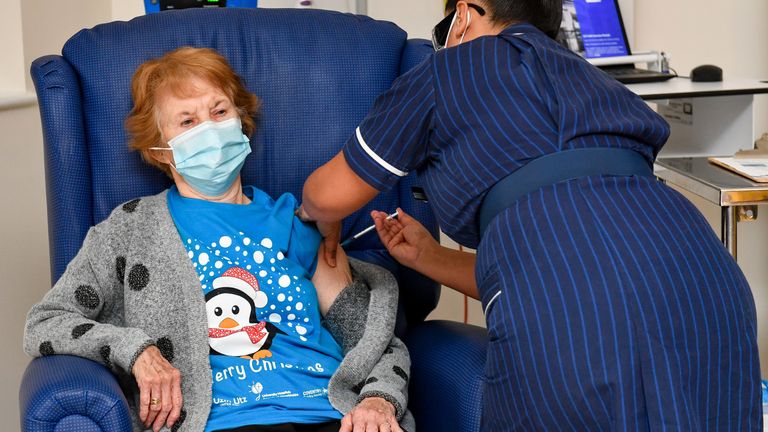
Four-year-old grandmother Margaret Keenan, 90, became the first person in the world to receive the job as part of a vaccination program.
Mrs Keenan, who has been in Coventry for six decades but is originally from Enniskillen, Northern Ireland, was vaccinated by May Nurse, a nurse at Perseus University Hospital.
He said the first was “a privilege” and “I wish him a happy birthday” because it meant he could spend most of the year with his family and friends in the new year after being his own.
Live COVID updates as UK Pfizer / Bioentech vaccine develops
He thanked Mrs. Parsons and other NHS staff for their “uninterrupted” care.
He added: “Someone I was offered the vaccine for – if I can keep it at 90, you can get it too!”
Mrs. Keenan, who only worked as a jewelry shop assistant until four years ago, will get a booster job in 21 days to ensure she has the best chance of being protected against the virus.
The UK was the first Pfizer Vaccine Approval Country Last week.
It started over the weekend The batch arrived at a hospital in south London Before the UK-wide rollout.
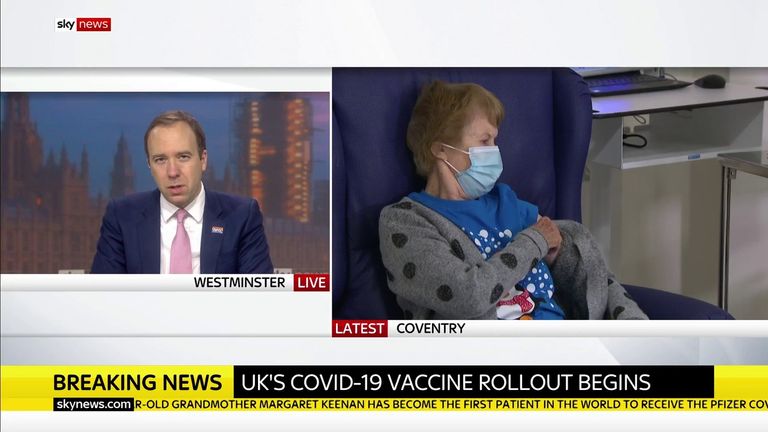
The vaccine, known as “V-Day” by Health Secretary Matt Hancock, will be administered at dozens of hospital centers from today – the first to receive the job is to take care of 60-year-olds and domestic workers.
Mr Hancock told Sky News he was “very emotional” to see Mrs. Kinen being vaccinated.
“It’s been a tough year for so many people and we’re finally moving forward with it – our light at the end of the tunnel …
“And just watching Margaret there – it seems very easy to have a run in your arm but it will protect Margaret and it will protect the people around her.
“And if we manage to do what is going to be one of the largest programs in the history of the NHS, if we manage to do this for everyone at risk of this disease, we can move forward.”
Nurse Mrs Parsons said it was the first “huge honor” in a country to supply this vaccine to a patient.
He added: “The last few months have been difficult for all of us working in the NHS, but now there seems to be light at the end of the tunnel.”
Originally from the Philippines, Mrs. Parsons has worked for the NHS for the past 24 years and has been at University Hospital Coventry and Warwickshire since 2003.
The government has protected 40 million doses of the vaccine, which need to be refrigerated at -0C (-94F). Studies have shown Pdo / BioNTech 95% effective in preventing jab COVID-19 And works in all age groups.
There could be as many as four million vaccines in the UK by the end of December, NHS providers told Sky News. About 800,000 doses came in the first batch.
Stephen Paus, NHS England’s medical director, said on Sunday that the CVVID vaccine should be introduced.The end seems to be the beginning“, But warned that the campaign would be a” marathon, not a sprint. “
Since the UK licensed the vaccine last week, the Medicines and Health Care Product Regulatory Agency’s (MHRA) regulatory approval process has drawn some criticism, suggesting it is speeding up public confidence.
Dr. Anthony Fawcett, Director of the US National Institute of Allergy and Infectious Diseases, Criticism of regulators It went so “fast” and “excessively” that America was “the gold standard of the regulatory system”. By comparison, he said: “The United Kingdom did not do it carefully.”
He later apologized and said his comments about the UK’s approval were “taken out of context”.
The issue of speedy approval of the MHRA was also published by the European Medicine Agency (EMA), which recommended that it is more likely to gain public confidence in its slow pace.
In addition to the vaccine rollout today, the government has released a report highlighting the work and success of its Vaccines Taskforce (VTF).
Boris Johnson said: “The approval of the Pfizer-Bioentech vaccine for use in the UK has been identified as an important step in our fight against CVV-19.
“But we still have some way to go and everyone has to follow the rules to keep the virus under control.”
The much-anticipated vaccine rollout this week came after more than 611,000 deaths from the virus in the UK as well as around the world – more than 1.5 million deaths worldwide.